Barrett’s Esophagus (BE) is a medical condition that highlights the intricate relationship between chronic inflammation and cancer. It typically arises as a response to prolonged gastroesophageal reflux disease (GERD), where the normal squamous epithelium of the esophagus is replaced by columnar epithelium. This change can lead to dysplasia, a precancerous alteration in cells. Crucially, dysplasia in BE is classified into two main categories: low-grade and high-grade. Understanding these distinctions is vital for patients and healthcare providers alike.
What is Dysplasia in Barrett’s Esophagus?
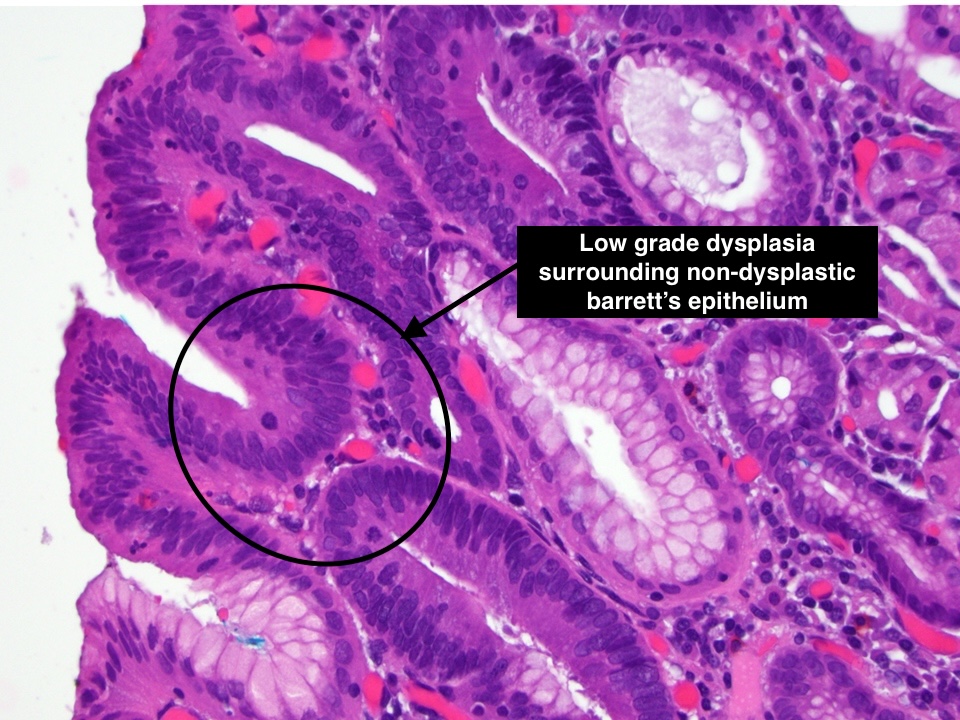
Dysplasia refers to abnormal cellular changes and is a key indicator of the potential for cancer development. In the context of BE, dysplasia is the transformation of the newly formed columnar cells into either low-grade or high-grade forms, each carrying different implications for cancer risk and management.
| Aspect | Low-Grade Dysplasia (LGD) in Barrett’s Esophagus | High-Grade Dysplasia (HGD) in Barrett’s Esophagus |
| Cellular Architecture | Mild disorganization. Cells maintain some structural order and resemblance to normal architecture. | Marked disorganization. Cells and glands show significant disruption in normal architecture. |
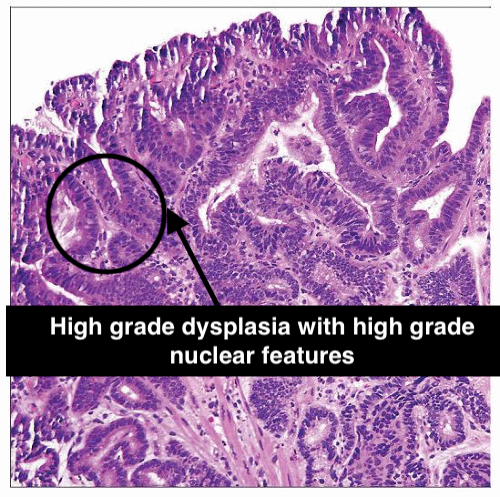
| Nuclear Changes | Slight enlargement and elongation of nuclei, but the changes are subtle. Nuclear hyperchromasia (increased nuclear staining) is mild | Prominent nuclear changes including significant enlargement, irregular shapes, and marked hyperchromasia. Nucleoli may be prominent. |
| Mitotic Activity | Low; mitotic figures (indicative of cell division) are infrequent and typically seen in the basal layer. | High; increased mitotic figures, including atypical forms, which may be present throughout the epithelium. |
| Glandular Features | Glands and crypts are relatively well-formed, although some irregularity may be present. | Glands and crypts are often irregularly shaped, fused, or cribriform (having a sieve-like appearance). |
| Risk of Progression | Lower risk of progression to adenocarcinoma compared to HGD. Progression, if it occurs, tends to happen over a longer period. | Significantly higher risk of progression to adenocarcinoma, often within a shorter time frame |
| Clinical Implications | Regular surveillance with endoscopy and biopsies to monitor for progression. Aggressive treatment is less common unless progression is observed. | Aggressive surveillance and treatment, including potential endoscopic resection or ablative therapies, due to the higher risk of progression to cancer. |
Helpful immunohistochemistry findings
- Usually not required for diagnosis, but many observers find p53 and Ki-67 labeling helpful
- Caution: p53 labels about 90% of HGD
- Subset is unlabeled
- p53 in LGD has correlated with progression to HGD in some studies
- Caution: p53 labels about 90% of HGD
- Routine H&E remains best “marker” of progression to cancer
Differential diagnosis of low grade and high grade dysplasia
Low-Grade Dysplasia
- Distinguish LGD from reactive changes
- Can be issue in patients taking colchicine or paclitaxel, which both result in mitotic arrest changes
- IFD category has obviated some problems with this distinction
- Has similar follow-up to LGD so this distinction not critical clinically
High-Grade Dysplasia
- Distinguish HGD from intramucosal carcinoma
- Somewhat subjective
- Nucleoli, intraluminal necrosis, and syncytial effacement of lamina propria are features of early intramucosal invasion
- On endoscopic mucosal resection samples, immunohistochemical more easily distinguished from HGD
- Duplicated muscularis mucosae can result in confusion about depth of invasion
- Finding ulcers associated with HGD raises possibility of unsampled invasive carcinoma
Treatment of Barrett’s Dysplasia and surveillance
- Indefinite for dysplasia:
- Repeat endoscopy after acid suppressive medication optimization for 3 – 6 months
- If indefinite for dysplasia is reconfirmed, surveillance interval of 12 months is recommended
- Low grade dysplasia:
- Endoscopic eradication therapy preferred treatment modality, endoscopic surveillance every 12 months an acceptable alternative
- High grade dysplasia:
- Endoscopic therapy
- Nodular Barrett esophagus: recommend endoscopic mucosal resection of the nodular lesion as initial diagnostic and therapeutic maneuver
- If endoscopic mucosal resection confirms high grade dysplasia, endoscopic ablative therapy of remaining Barrett esophagus
- Nonnodular dysplastic Barrett esophagus: radiofrequency ablation currently preferred endoscopic ablative therapy
- In patients with preablation low grade dysplasia, endoscopic surveillance recommended every 6 months in the first year following complete elimination of intestinal metaplasia (CIEM) and annually thereafter
- Endoscopic surveillance following complete elimination of intestinal metaplasia for patients with preablation high grade dysplasia recommended every 3 months for the first year, every 6 months in the second year and annually thereafter
Histopathological evaluation is crucial in distinguishing between LGD and HGD in Barrett’s Esophagus. This evaluation guides clinical management, with more aggressive surveillance and treatment for HGD due to its higher risk of progression to cancer.
Solve some MCQs on the topic
- What is a characteristic histological feature of low-grade dysplasia in Barrett’s esophagus? a. Glandular crowding and irregularities b. Normal cellular morphology c. Squamous metaplasia d. Presence of goblet cells
- How is low-grade dysplasia in Barrett’s esophagus typically managed? a. Immediate surgery b. No intervention, only regular surveillance c. Radiotherapy d. Chemotherapy as the primary treatment
- What feature helps distinguish high-grade dysplasia from early-stage adenocarcinoma in Barrett’s esophagus? a. Absence of dysplastic changes b. Invasion into the submucosa c. Decreased nuclear atypia d. Presence of normal glandular architecture
- What is a common treatment approach for patients diagnosed with high-grade dysplasia? a. Endoscopic mucosal resection (EMR) b. No intervention, only close observation c. Systemic chemotherapy d. Esophagectomy as the primary option
Answers:
- Correct Answer: a. Glandular crowding and irregularities
- Correct Answer: b. No intervention, only regular surveillance
- Correct Answer: b. Invasion into the submucosa
- Correct Answer: a. Endoscopic mucosal resection (EMR)
References
- Sternberg diagnostic surgical pathology 7th edition]
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7684982/
Join our approach based course for useful tips, pitfalls, notes, reporting templates and worksheets- find details below.
More approach based algorithms in the course- Approach to oesophageal biopsies non-neoplastic based on various patterns, approach to gastric biopsies based on patterns, approach to spindle cell lesions of the GIT, approach to small intestinal biopsies based on patterns and approach to colonic biopsies including inflammatory bowel diseases.
Leave a Reply